Past Research
Dr. Hasyim’s past research was primarily focused on experimental work, spending considerable time in the laboratory fabricating materials and energy devices, and developing mathematical models to understand their behavior. This hands-on approach provided him with valuable insights into the practical challenges of materials science and electrochemistry, forming a strong foundation for his current theoretical and computational work.
Whether it’s a battery or supercapacitor, an energy device must overcome transport and electrochemical barriers, inherent in their materials design, to deliver suitable power and energy density demand. During his undergraduate studies, Dr. Hasyim had the opportunity to fabricate, test, and mathematically model these devices.
All works on electrochemical capacitors were done under the supervision of Prof. Ramakrishnan Rajagopalan. All works on solid electrolytes were done under the supervision of Prof. Michael T. Lanagan, with close mentorship of Dr. Seth Berbano. Additional supervision was also done under Regis Cleary and Prof. Dinesh Agrawal.
Experimental Studies and Modeling of Electrochemical Capacitors
Dr. Hasyim and collaborators developed a methodology, coined Frequency Domain Admittance Method (FDAM) for analyzing electrochemical capacitors [1,2] . The method utilizes linear impedance data, which is rich in microscopic and macroscopic information, to predict energy and power density of a capacitor.
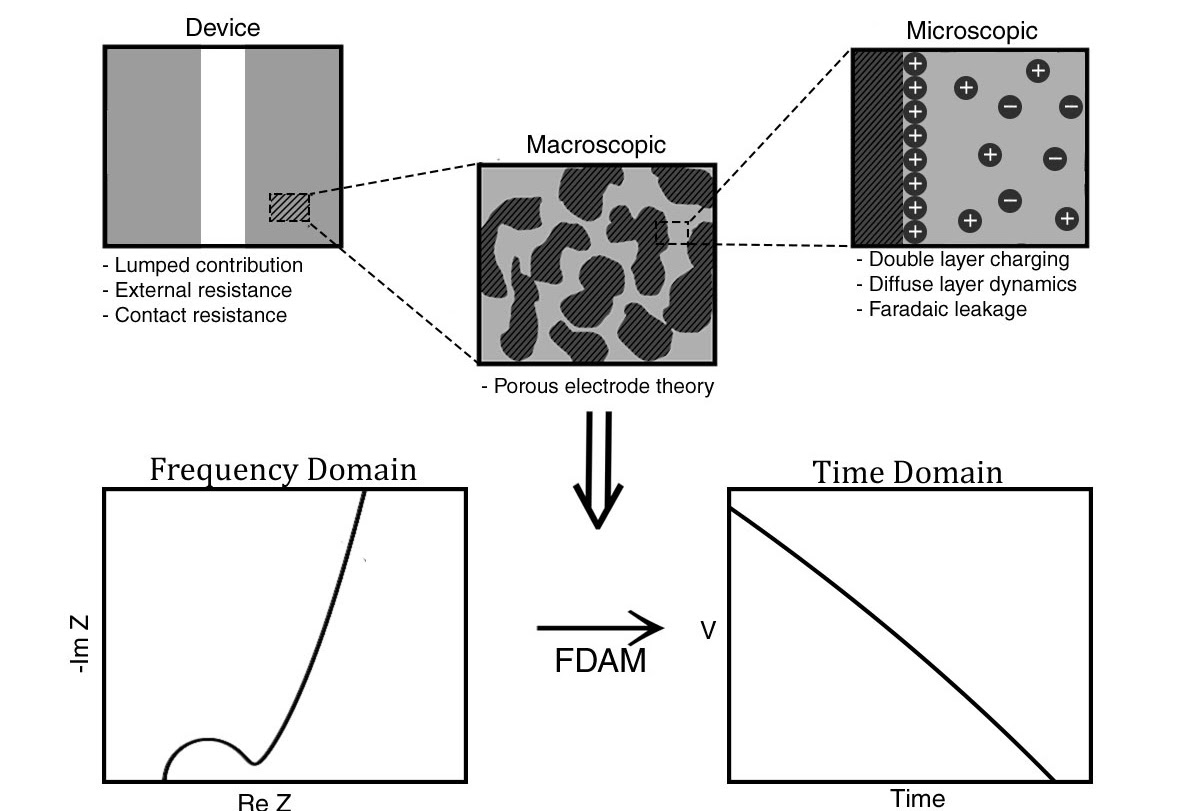
FDAM was first tested to electrical double layer capacitors (EDLCs) [1] fabricated by Danhao Ma as well as commercial ones with great success. Afterwards, the research team tested their methodology to \(\mathrm{MnO}_2\) and poly-pyrrole psuedocapacitors [2] that Dr. Hasyim fabricated with similar success.
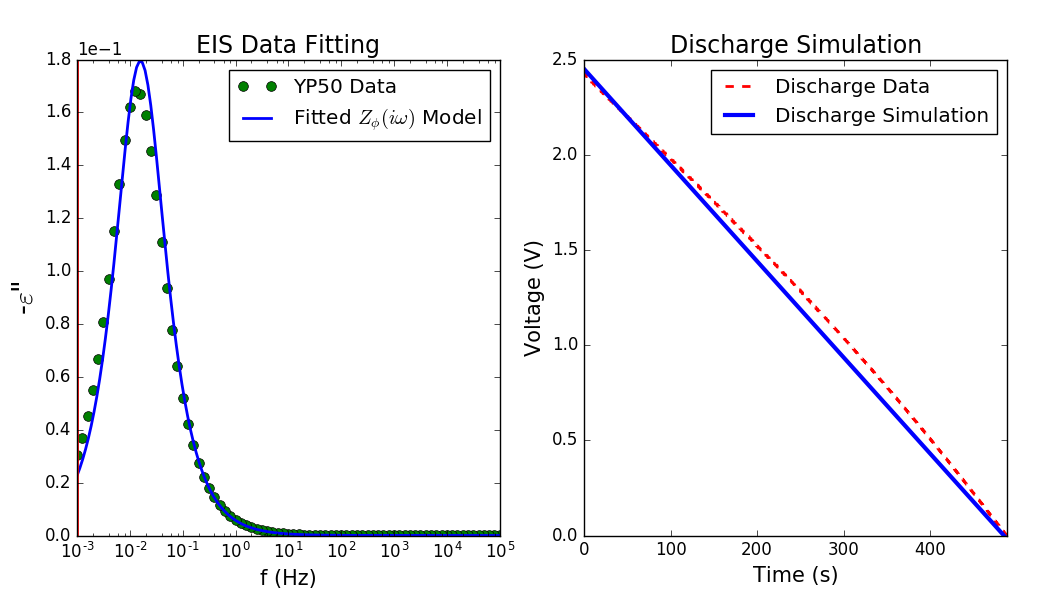
The code supplementing this work is available as a Python Library on GitHub (PyFDAM). Paper on EDLCs and pseudocapacitors can be found in Ref. [1] and [2], respectively. Both works contribute to Dr. Hasyim’s thesis (Link).
Experimental Studies and Modeling of Composite Solid Electrolytes
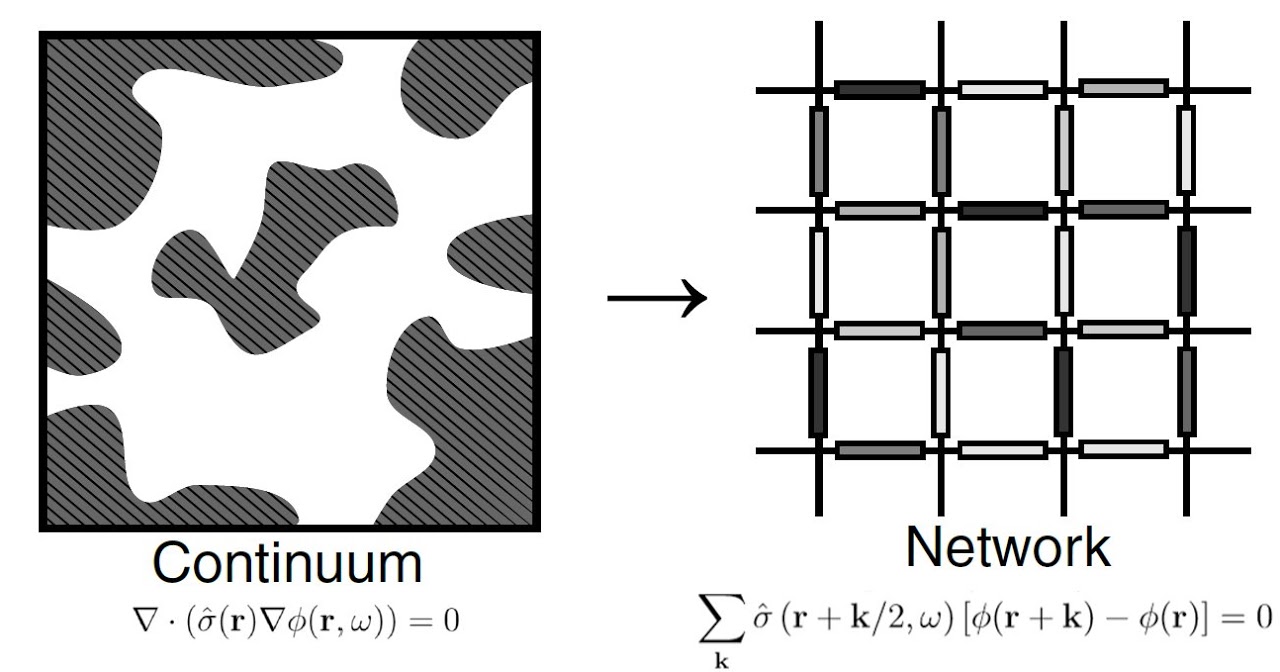
Dr. Hasyim and collaborators fabricated lithium-borate-silica composite electrolytes and characterized their impedance characteristics and microstructure [3]. Motivated by the broad frequency response of these materials, the research team developed a percolation model [4] for a multi-component solid electrolyte based on the effective medium approximation, which can explain both DC and AC conductivity of various composites.
Paper for the experiments and modeling can be found in Ref. [3] and [4] . Both works contribute to Dr. Hasyim’s thesis (PDF).