Molecular Polaritonics
Molecular polaritonics is an emerging field that explores how material and molecular properties can be modified by strong coupling between molecules and confined light fields in optical cavities. When molecules are placed between two highly reflective mirrors forming a cavity, their electronic or vibrational transitions can strongly interact with the cavity’s electromagnetic field modes. This interaction leads to the formation of hybrid light-matter states called polaritons. The field combines concepts from quantum optics, physical chemistry, and materials science.
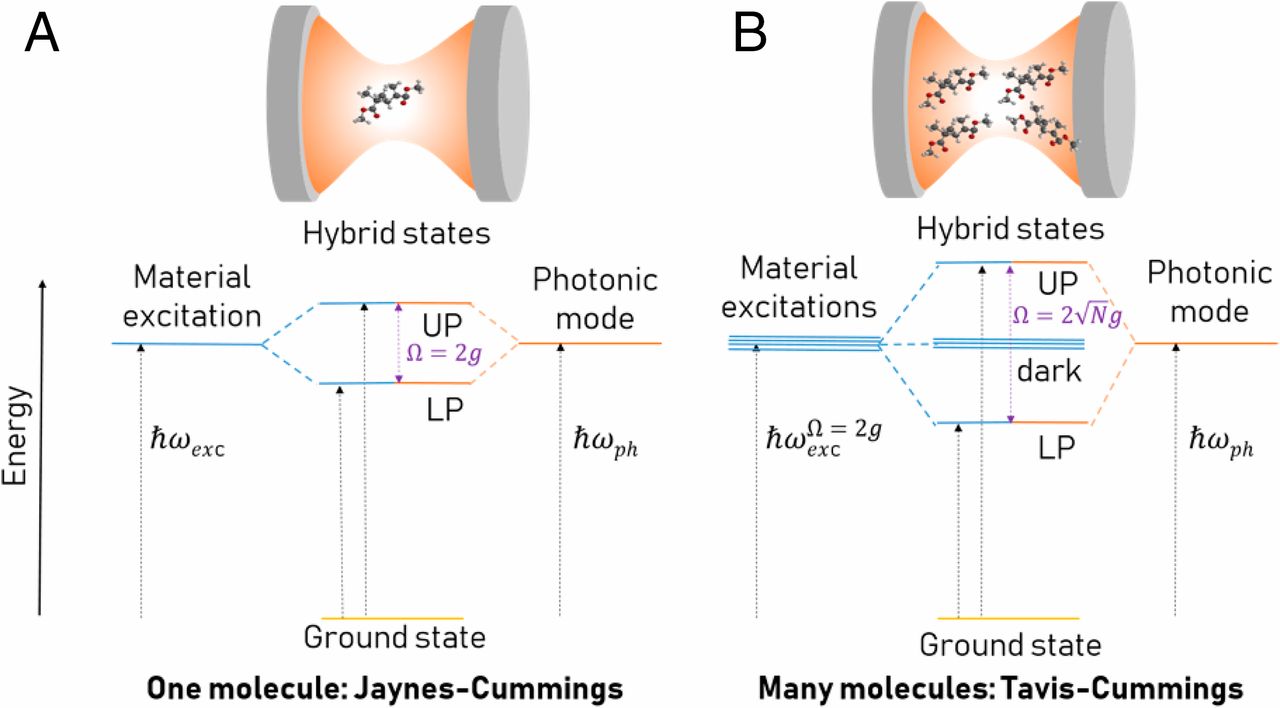
The formation of polaritons can dramatically alter the energy landscape and dynamics of chemical systems, enabling:
- Modification of reaction rates and pathways
- Control over energy transfer processes
- Enhanced charge separation in photovoltaic materials
- Development of novel chemical sensors
- Creation of quantum materials with unique properties
Dr. Hasyim’s research focuses on developing theoretical frameworks and computational methods to understand and predict how strong light-matter coupling affects chemical processes at both the microscopic quantum level and macroscopic ensemble level. All works are in collaboration with Prof. David R. Reichman, Prof. Arkajit Mandal, and Prof. Norah Hoffmann.
Towards Accurate Mixed-Quantum Classical Simulations of Polaritonic Chemistry
A key challenge in studying molecular polaritonics is accurately simulating the quantum dynamics of many molecules strongly coupled to confined light modes. While fully quantum mechanical calculations are possible for single molecules, they become computationally intractable when modeling the collective effects of multiple molecules - a crucial regime for understanding real experimental systems.
Mixed quantum-classical (MQC) methods offer a promising middle ground, treating the most important quantum effects while using classical approximations where appropriate to maintain computational feasibility. However, even for single-molecule systems, traditional MQC approaches have important limitations that need to be addressed.
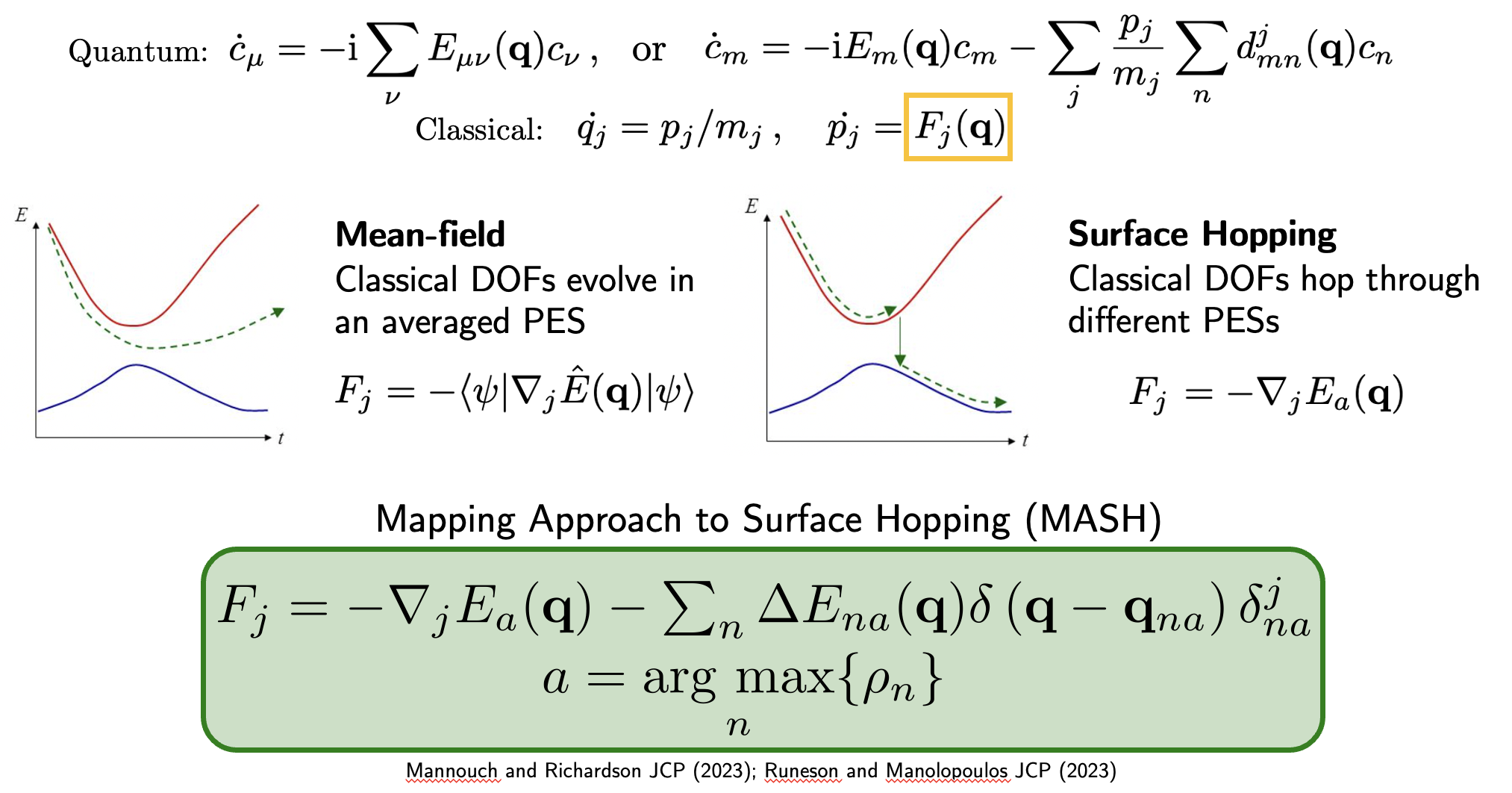
The work makes two key advances:
Implementation of the Mapping Approach to Surface Hopping (MASH) - an improved MQC method developed by Dr. Jonathan Mannouch and Prof. Jeremy Richardson, refined further by Dr. Johan E. Runeson and Prof. David Manolopoulos that better captures quantum effects compared to conventional approaches
Incorporation of a quantum treatment of the cavity mode itself, moving beyond the classical approximations typically used in previous studies
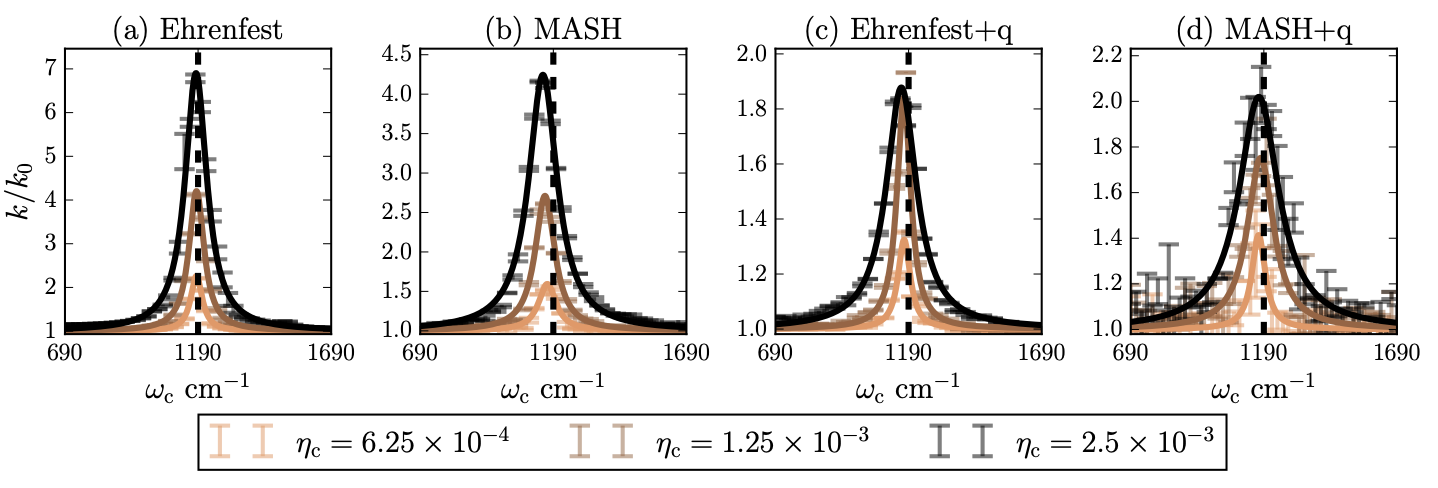
Their results show that combining MASH with a quantum cavity description provides the most accurate reaction rates in single-molecule test cases. However, the research team discovered that this approach can face challenges with “size-inconsistency” - where the dynamics at zero coupling depend on whether the cavity mode is quantized.
To address this, Dr. Hasyim and collaborators developed ε-MASH, which prevents unphysical transitions between states with negligible coupling. This modification maintains accuracy while ensuring proper behavior in important limiting cases.
These methodological advances lay the groundwork for accurate and computationally tractable simulations of collective effects in molecular polaritonics - a crucial step toward understanding and predicting how strong light-matter coupling can be harnessed to control chemical reactivity.
Paper can be found in Ref. [1]